H. P. Friedrichs (AC7ZL) Homepage
Radio Room
The Crystals of Crystal Radio
Note: This article first appeared in the September 2014 issue of the Xtal Set Society newsletter.
Introduction
"Crystal Radio." The very term for this technology contains, embedded within it, reference to the iconic material responsible for extracting intelligence from the waves in the aether--the detector crystal. Sadly, it seems that most contemporary crystal set builders interpret this word to mean a factory-made 1N34 or 1N60 germanium diode. Even among adventuresome builders, those who might craft their own crystal cups and cat's whisker mechanisms, rarely is attention paid to natural detector materials outside of galena.

Galena (Image courtesy of Wikipedia)
In truth, there is a wide variety of metallic minerals that exhibit useful electrical properties, and in the early days of radio many of them were pressed into service.
What is a crystal detector? In essence, it is a primitive semiconductor, not unlike the silicon or germanium-based diodes found in modern electronics, except that they are usually comprised of naturally-occurring--as opposed to human-engineered--materials. Detector behavior emerges when pairs of materials, two different types of crystal for example, or a crystal and a wire probe, are brought into intimate contact. A complete detector includes not only the raw materials just mentioned, but some kind of adjustable mechanical contrivance used to position and hold the semi-conducting materials against one another.
A Guide to Crystal Detector Data
So which naturally-occurring materials make for a good detector? Table 1 contains a list of possible candidates that I've assembled. The list is by no means exhaustive, but it does draw from multiple sources including the October 1925 edition of J.F. Corrigan's Crystal Experimenter's Handbook, Ralph Stranger's 1928 work Wireless The Modern Magic Carpet, Alfred Morgan's 1913 Wireless Telegraph Construction for Amateurs, Chris Pellant's 1992 Rocks and Minerals, and assorted books and materials from my personal library.
The first column in the table contains the names of various mineral types. Note that some minerals are known by more than one name, and if I could determine that, I included references to both. I did not acknowledge period references to "inserite," "tserine," or "iserine" because, frankly, I could find no mention of them in any of the more modern books that I have. As I am not a geologist, I'll accept fault for their exclusion. Maybe these particular names are no longer in vogue or the substances in question are now known by different, newer names.
Some substances were flagged by Corrigan as being commercially successful detector materials. These I've signified in the table with a bold font.
The next three columns tell us something about the chemical composition of each mineral. Column two provides a chemical name, column three specifies a chemical formula, and column four classifies the compound. In extracting data from period sources I noticed that some of the chemical information was in minor disagreement with more modern sources. In those cases, I defaulted to the more recent data.
Column five is of particular interest, because it says something about what combinations of materials will result in useful detectors. This information was taken primarily from Corrigan.
Column six reflects specific references to minerals made by Corrigan in his Crystal Experimenter's Handbook, mentioned earlier. An "X" appears in the row of any mineral described by that book. Column seven reflects the recommendations of a Dr. D.H. Eccles who is quoted in Stranger's Magic Carpet. Column eight represents the recommendations of Japanese scientist Wichi Torricata whose comments were published in the September 16, 1910 edition of Electrician. The Electrician piece, incidentally, is also cited in Stranger's book. Column nine represents references in Alfred Morgan's work.
Thoughts on Various Detector Crystals
It comes as no surprise that galena is on the list, and is endorsed by four authors. Because my own experience with galena detectors involves crystals probed with the classic wire "cat's whisker," it was interesting to discover that a galena detector can also be built with galena-on-galena, silicon-on-galena, and graphite-on-galena. In the latter case, I can envision probing the galena crystal with a soft (low clay content) #1, #2 or B-grade pencil lead.
My favorite whisker material for galena is phosphor bronze wire. As a guitar player, I enjoy a ready supply of phosphor bronze wire in the form of used acoustic guitar strings. In guitar strings, the bronze wire is wound around a core of steel music wire to thicken the string and reduce its pitch, generally the low "E," "A," and "D" strings. Extraction of the bronze wire involves no more than unwrapping a segment of guitar string to remove whatever length of bronze wire is needed.
Speaking of pencil leads, any crystal radio builder worth his salt has heard of the so-called "foxhole" radio. First described in the July 1944 issue of QST magazine, the foxhole radio was a primitive crystal receiver devised by clever soldiers stationed on the Anzio beachhead. Their detector was comprised of a blued razor blade probed by a piece of pencil lead. Chemically speaking, the bluing is a form of magnetite and pencil lead is, of course, predominantly graphite. Both of these materials are represented in Table 1.
Also to be expected, iron pyrites appear in the table. Iron pyrite, or "fool's gold", is a readily-available and cheap mineral to purchase and play with. I have found that while not all samples make effective detectors, most do. My favorite configuration involves the pyrite crystal probed with a steel wire whisker. Music wire with its high carbon content is nice and springy, and can also be harvested from guitar strings (I suggest the 0.008-0.010-inch high "E" strings). I provide detailed plans for a pyrite detector in Chapter 11 of my book, The Voice of the Crystal.
Another detector material that I have some experience with, which appears in Table 1, is cuprous oxide. I've written extensively on experiments with this substance in my book Instruments of Amplification, and my article "Fun With Homebrew Cuprous Oxide Diodes" which appeared in the January 2010 issue of the Xtal Set Society Newsletter. The mineral form of cuprous oxide is called cuprite. In the case of my experiments, the oxide I used was created artificially on the surface of carefully-prepared samples of copper metal.
Table 1 suggests tellurium and antimony as suitable contacts for use with cuprite. Homegrown cuprous oxide films, however, are fragile and are subject to unintentional scratches or penetration. Thus, my favorite probe material for cuprous oxide is metallic lead or solder. Unlike harder metals like bronze or steel, lead is soft and compliant. As well, I've experimented with tiny beads of indium and even dots of silver-bearing ink. All work well as contact materials. Though I haven't tried it, graphite might be worth a look.
Given my own success with copper oxides, I find chalcocite, or copper sulfide, an intriguing variation. I've never played with chalcocite, but Corrigan's handbook describes a technique for making your own copper sulfide detector.
One must carefully melt a small quantity of sulfur until it's fully liquefied. The end of a copper rod, previously cleaned and polished to a bright shine with emery paper, is immersed in the liquid for several minutes, and then withdrawn. Finally, the coated end of the rod is ignited, and any free sulfur that remains is allowed to burn off. Corrigan reports that the coating thus formed works well in contact with zincite, or even when probed by the usual cat's whisker. The author does not mention what I feel I must: Such preparations will surely result in the production of toxic fumes, so experiments like this should be conducted outdoors, and then only while wearing suitable protective gear like leather gloves and safety glasses.
If one mineral can be said to dominate Table 1, that would be zincite. Zincite, it seems, will produce a useful detector junction with just about everything else. Zincite in contact with a copper pyrite crystals form the basis of G.W. Pickard's legendary "Perikon" detector.
Zincite has the additional property that, if stimulated properly, it can be induced to both amplify and to oscillate-- the very properties that make modern transistors so useful. It is a pity that much of the interest in crystal oscillators vanished when practical vacuum tubes first appeared. However, contemporary experimenters like Nyle Steiner have rediscovered and written about this phenomenon.
Given the random processes responsible for natural crystal formation, the specific chemical content of a given mineral type is subject to variability imposed by the whims of nature and chance. For example, if foreign substances are present when minerals are being formed, those compounds may be integrated into the emerging crystals, thus modifying their physical properties. In crystal radio terms, this can result in the non-responsive or "dead" detector mineral that early radio enthusiasts occasionally grumbled about. However, sometimes the type and concentration of the adulterants is such that they actually enhance the signal-detecting properties of the crystal. Two examples of this readily come to mind.
Galena, chemically speaking, is a lead sulfide. However, my readings suggest that the best galena for crystal radio detectors is not pure, but a type which contains trace amounts of silver. This latter type of galena, called argentiferous galena, is said to produce superior detectors, both in sensitivity and stability.
Another example is zincite. Zincite is a simple oxide of the metal zinc. Pure synthetic zincite crystals are typically clear and colorless, but from what I've gathered, the zincite with the most desirable radio properties is red. This red color manifests itself in the zincite crystals because they've been naturally tainted with manganese and iron.
Incidentally, modern semiconductor manufacturers make routine use of contaminants in their products, albeit in an intentional and carefully-controlled manner. The purpose of this is to beneficially alter the electrical properties of the materials from which transistors and integrated circuits are made. The industry calls this practice doping.
Ideal Detector Crystals
A complete list of naturally-occurring detector materials would far exceed the contents of Table 1. However, when it comes time to apply these materials to an actual radio receiver, certain practical considerations immediately shrink the size of any pool of candidates.
For example, some minerals are comparatively rare or exhibit detrimental chemical properties. Despite Torricata's endorsement, sylvanite is a good example of both. It is a fairly rare crystal which tends to makes it very pricey. Then, some instances of sylvanite are actually photosensitive and will tarnish upon exposure to light.
An ideal detector should be easy to adjust and set into action. Once set, the detector should remain functional over a useful interval of time. Yet, success with many natural detector materials depends upon the pressure with which the crystals are probed. Some require robust pressure between crystals or the crystal and its whisker. Other detector materials will function with the slightest touch. Some are so sensitive to mechanical shock that it's difficult to get them properly adjusted, and once so, a hard stare is sufficient to knock them out of whack. Despite otherwise useful properties, such unstable materials will result in a detector that is more trouble than it's worth.
It is best if a detector is "sensitive." Sensitivity is a consequence of the voltage that must be applied to the crystal in order to get it to conduct, and the rate at which current through the crystal rises with further increases in applied signal. Why is this important? Because if we attempt to detect a radio signal whose amplitude lies below the threshold of conduction for the detector, the detector will remain in an "off" condition and the signal will not be heard.
Let's talk about carborundum in the context of these last few paragraphs. Carborundum is a synthetic industrial abrasive. It doesn't have to be mined, and there is no shortage of it because it's easy to make. Consequently, carborundum is relatively easy to obtain and its price is reasonable.
Carborundum is normally probed with steel, another common material. Carborundum likes firm contact between itself and the steel probe, which lends itself to the construction of mechanically stable detectors. Once adjusted, a carborundum detector tends to stay properly adjusted for a long time.
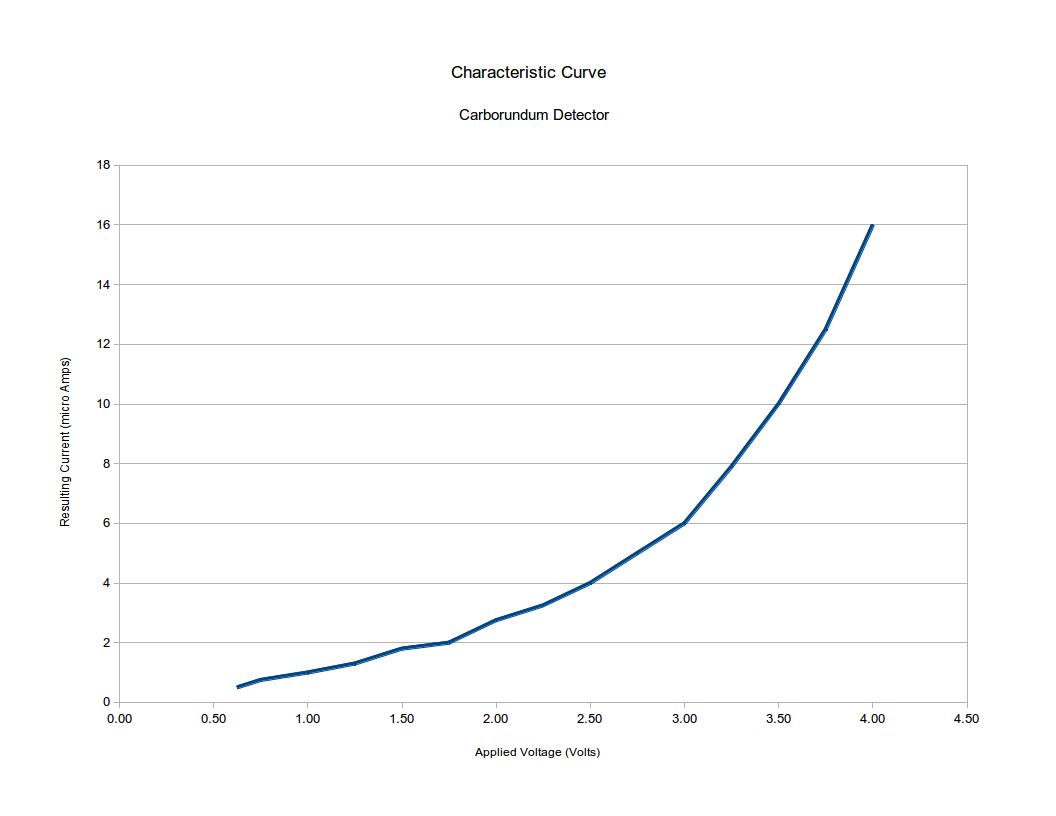
Figure 1: Response curve for carborundum detector
Sometimes a Little Bias is a Good Thing
Regrettably, carborundum is not a very radio-sensitive material. Whereas a galena detector or a germanium diode like the 1N34 will begin conducting signals as small as a few tenths of a volt in amplitude, a carborundum detector will not function properly until the applied signal reaches a volt or more--a tenfold reduction in comparative sensitivity--which renders it useless for weak-signal work. Figure 1 is a graph adapted from Bucher showing the response curve for a typical carborundum crystal. Given this apparent lack of sensitivity, why is carborundum bolded in Table 1, signifying it as a commercially successful detector material?
It turns out that carborundum's problems can be mitigated through the careful application of what is called a "bias voltage." The idea is to use a small battery to apply an electrical potential on the detector that is almost, but not quite, sufficient to force it into conduction. Operating the crystal in this manner means that a radio signal superimposed on the bias, even a very tiny signal, will then be sufficient to trip the crystal into conduction. It should be understood that the same technique can be applied to other detectors to varying degrees of advantage.
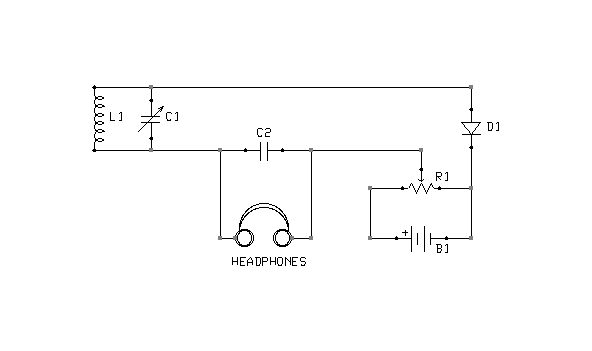
Figure 2: Bias arrangement for Marconi Model 107A Tuner
Figure 2 depicts a simple crystal receiver with an option to bias its detector. The circuit was adopted from the Marconi Model 107A Tuner.
The biasing circuitry consists of nothing more than a couple of dry cells in series (B1) and a potentiometer (variable resistor, R1). When the potentiometer is at its lowest setting (to the far right), battery current flows through the potentiometer only. No potential is applied to the detector (D1). As the potentiometer is advanced to the left, however, an increasing voltage will be applied to the detector through a circuit completed by the headphones and tuning coil (L1). Figures 3, 4, and 5 depict minor variation on this basic idea.
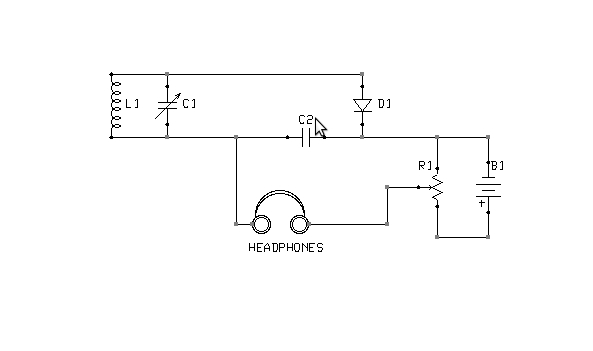
Figure 3: Alternate biasing arrangement
What is the correct point of adjustment? That depends upon the detector materials in use and is best determined through trial and error. Too much bias will render a detector as deaf as no bias at all. Note also that it is possible to bias a detector in the wrong direction. If advancing the potentiometer does not result in increased sensitivity, the battery terminals should be swapped, so as to reverse the polarity of the bias voltage. The circuit shown in Figure 5 is interesting in this context, because it is capable of applying either a positive or negative bias, of varying magnitude, without rewiring the battery.
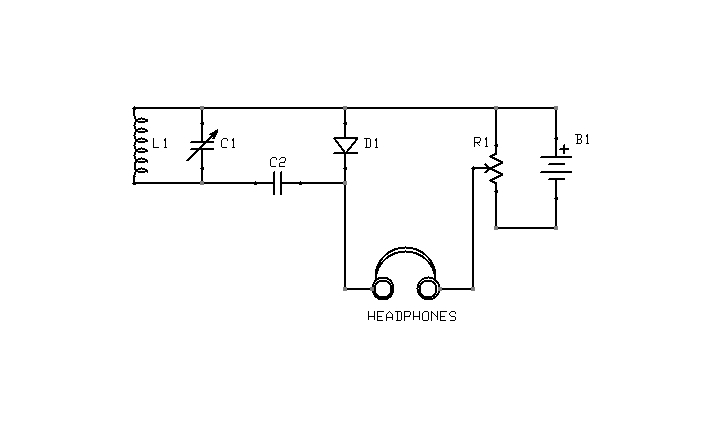
Figure 4: Yet another biasing arrangement
Conclusion
Where can one find samples of minerals to experiment with? I checked the phone directory listings for a half-dozen major cities in the United States, and each revealed several rock and mineral shops where detector materials might be purchased. If brick-and-mortar shops are not your cup of tea, minerals are offered for sale through numerous Internet retailers including giants like Amazon and Ebay.
A Google search of the phrase "minerals for sale" yields more than ten million hits. In this day and age, the Internet may be the best place to begin any search of this type.
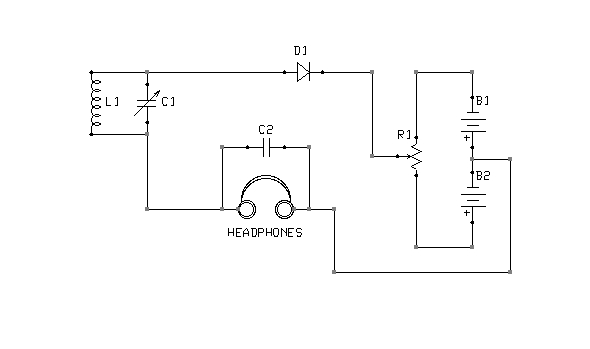
Figure 5: Bipolar biasing
I am fortunate to live in Tucson, Arizona, host of the annual International Gem and Mineral Show. Each year, hundreds of vendors converge on our city to offer for sale a staggering variety of mineral specimens, collected from the four corners of the earth. This event takes place between mid-January and mid-February of each year. If you happen to be the inhabitant of a state or province that is normally snow-bound at that time of year, the search for radio detector minerals can provide a wonderful excuse to visit and enjoy Tucson's warmth and hospitality.
If all else fails, poke around in the dirt in your backyard, at the park, a hiking trail, or on the beach. You might be surprised what you find.
Below is a list of some of the information sources cited in this article. I also invite you to visit my website at: www.hpfriedrichs.com
Bibliography
Bucher, Elmer. Practical Wireless Telegraphy. New York: Wireless Press, 1921.
Corrigan, J.F. Crystal Experimenter's Handbook. ??: Popular Wireless, 1925.
Coursey, Philip. Telephony Without Wires. London: The Wireless Press Ltd, 1919.
Friedrichs, H.P. Instruments of Amplification. Tucson, AZ: H.P. Friedrichs, 2003.
Friedrichs, H.P. The Voice of the Crystal. Tucson, AZ: H.P. Friedrichs, 1999.
Gabel, Victor. "The Crystal as a Generator and Amplifier." Wireless World & Radio Review. October 1, 1924:p2
Garten, Justin. "Splatter." QST Magazine. October, 1944:p86
Kujanpaa, Toivo. "Strays." QST Magazine. July, 1944:p62
Lange, Norbert Adoph. Handbook of Chemistry. Sandusky, OH: Handbook Publishers, 1956.
Morgan, Alfred. Wireless Telegraph Construction For Amateurs. New York: D. Van Nostrand Co., 1913.
Pellant, Chris. Rocks and Minerals. New York: DK Publishing, 1992.
Stranger, Ralph. Wireless The Modern Magic Carpet. London: Partridge Press, 1928.
Toricata, Wichi. "Commerical Wireless Telegraphy in Japan." Electrician. September 16, 1910:p??
Document Pilot 11/13/2014
Revised 05/12/21



